
GLP-2T
$130.80 – $339.60Price range: $130.80 through $339.60
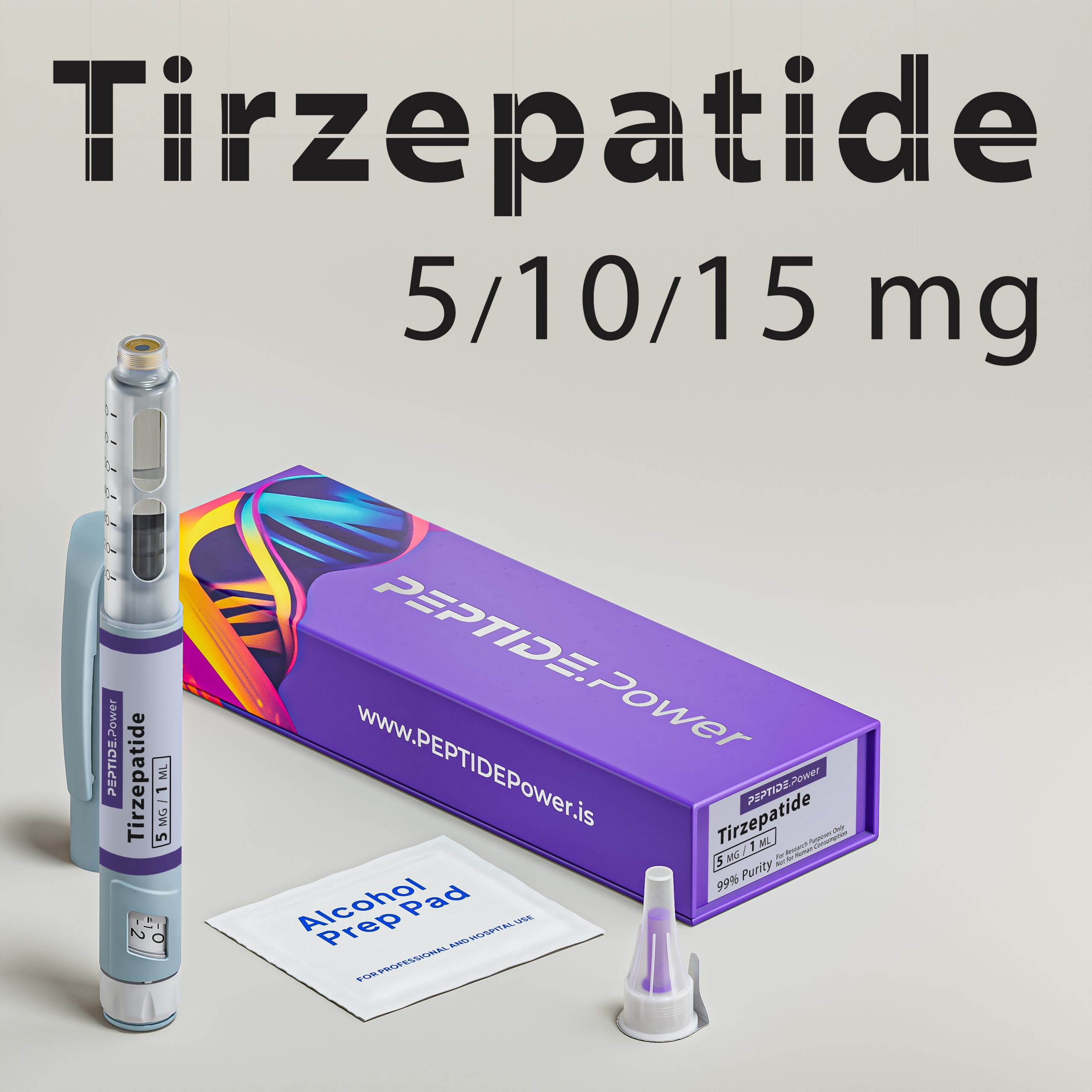
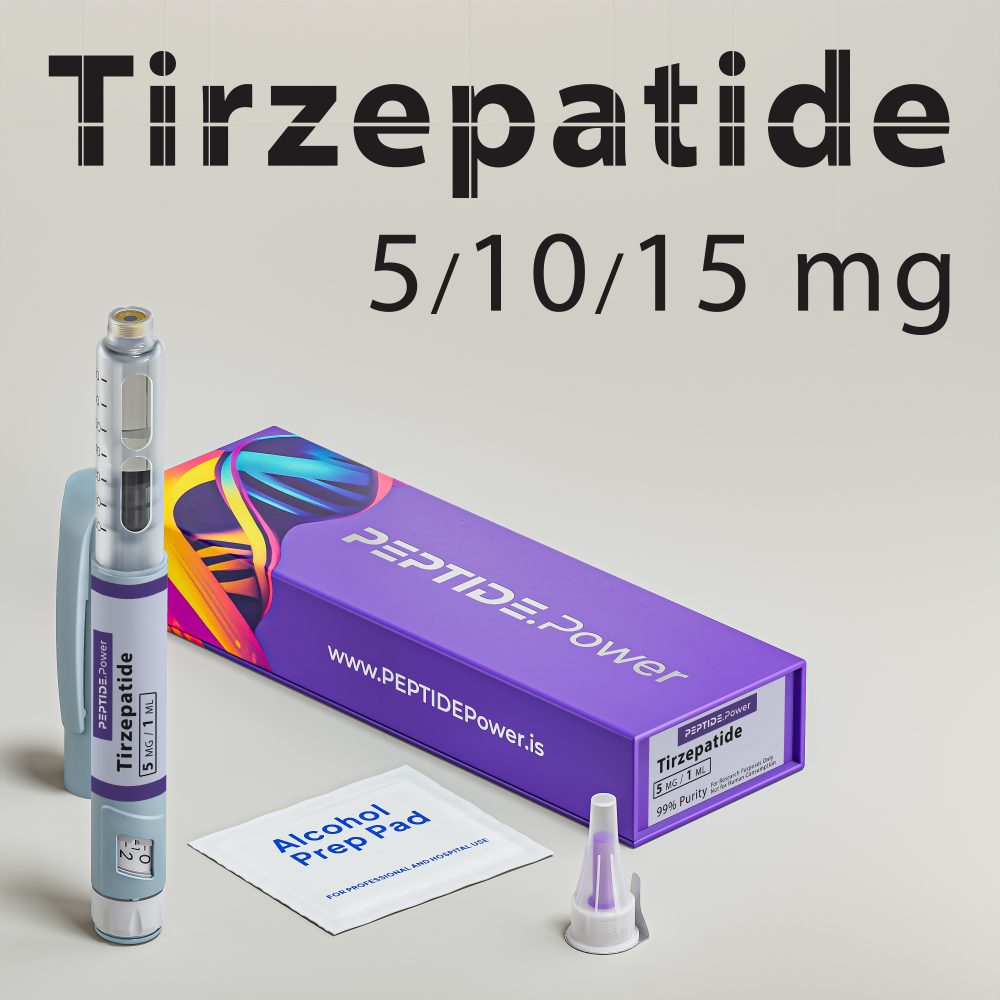
Tіrzеpаtіdе - Dual GLP-1/GIP Agonist for Weight Loss & Metabolic Support
Description
Tіrzеpаtіdе is a synthetic analog of the natural hormones GLP-1 and GIP, designed for research use in metabolic and obesity studies. Originating from pharmaceutical development, this peptide targets metabolic regulation, glucose balance, and fat metabolism.
In research models, Tіrzеpаtіdе has been observed to enhance insulin secretion and reduce appetite, supporting energy balance and metabolic efficiency.
Formulated in a stabilized pre-mixed injection pen for subcutaneous use, it provides high bioavailability and ease of dosing in experimental settings.This peptide is intended for research only and not for human use. Clinical studies have shown notable effects on weight and glucose control, making it one of the most promising peptides in metabolic research.
Clinical Status:
FDA-approved for type 2 diabetes and chronic weight management (brand formulations); other uses remain research-only.
Evidence Type:
Human RCT ✔ | Observational ✔ | Animal ▣ | In vitro ▣ | Regulatory ✔
Mechanism of Action
Tіrzеpаtіdе binds to GLP-1R and GIPR on pancreatic β-cells, enhancing glucose-dependent insulin release and inhibiting α-cell glucagon secretion. The synergistic dual-agonist action improves hepatic glucose output, lipid oxidation, and mitochondrial efficiency. Additionally, central appetite regulation via hypothalamic POMC/CART activation results in reduced caloric intake.
Benefits
- Dual Agonist Mechanism for Metabolic Optimization:
Tіrzеpаtіdе is studied as a dual agonist of the GLP-1 and GIP receptors, providing simultaneous stimulation of two key incretin pathways that regulate insulin secretion, appetite, and energy balance. This dual mechanism enables superior glucose regulation and energy expenditure compared to single agonists, making it one of the most advanced compounds in metabolic peptide research. - Significant Weight Reduction in Research Models:
In human and animal studies, Tіrzеpаtіdе has demonstrated marked body weight reduction through decreased caloric intake and enhanced lipid metabolism. Reported findings indicate up to 22% average weight loss in long-term trials, associated with improved metabolic flexibility and reduced visceral fat accumulation. These effects are of high interest for obesity and body composition research. - Improved Glucose Control and Insulin Sensitivity:
Tіrzеpаtіdе enhances pancreatic insulin secretion and suppresses glucagon in a glucose-dependent manner. It has been observed to lower fasting glucose and HbA1c levels significantly in both diabetic and non-diabetic research participants. These improvements stem from coordinated incretin receptor activation that improves beta-cell function and peripheral insulin sensitivity. - Reduction of Visceral and Hepatic Fat:
Preclinical and clinical data demonstrate that Tіrzеpаtіdе reduces visceral adipose tissue and liver fat content by modulating lipid oxidation and suppressing de novo lipogenesis. This mechanism contributes to reduced hepatic steatosis and improved metabolic liver function, positioning it as a leading peptide in experimental models of fatty liver and metabolic syndrome. - Enhanced Lipid Profile and Cardiometabolic Benefits:
Research outcomes indicate a significant reduction in total cholesterol, LDL, and triglycerides with concurrent elevation of HDL levels. Tіrzеpаtіdе’s effect on lipid metabolism and vascular function supports its potential use in studies focused on cardiovascular health and long-term metabolic optimization. - Regulation of Appetite and Satiety Hormones:
By modulating hypothalamic pathways and gut hormone signaling, Tіrzеpаtіdе influences appetite control and satiety perception. This leads to lower caloric intake without inducing compensatory hunger or muscle catabolism. Such behavioral and neuroendocrine modulation is a critical area of ongoing research in weight regulation and eating behavior studies. - Improved Energy Expenditure and Mitochondrial Efficiency:
Studies indicate that Tіrzеpаtіdе increases energy expenditure by activating thermogenic processes in brown adipose tissue and enhancing mitochondrial efficiency. These outcomes reflect a systemic improvement in metabolic energy flow, supporting its inclusion in research on metabolic performance and energy utilization optimization. - Reduction of Inflammatory Markers:
In metabolic research models, Tіrzеpаtіdе administration has been associated with decreased levels of inflammatory cytokines such as CRP, TNF-α, and IL-6. This anti-inflammatory effect is attributed to improved insulin sensitivity and lipid metabolism, which collectively reduce metabolic stress and systemic inflammation. - Preservation of Lean Muscle During Weight Loss:
Unlike many calorie-restriction interventions, Tіrzеpаtіdе supports the maintenance of lean muscle mass during fat reduction. This effect is vital in research evaluating sustainable body recomposition and metabolic health, as it preserves basal metabolic rate and muscular strength while decreasing total adiposity. - Synergistic Effects with Other Metabolic Peptides:
Experimental designs often explore the combination of Tіrzеpаtіdе with Cagrilintide, Sеmаglutіdе, or AOD-9604 to evaluate enhanced effects on lipid metabolism, appetite regulation, and glucose balance. These synergistic models aim to amplify fat loss outcomes and expand understanding of dual and triple incretin receptor activation in metabolic research. - Long-Term Metabolic Adaptation:
Extended-duration studies reveal that Tіrzеpаtіdе maintains metabolic improvements beyond the treatment period, suggesting sustained changes in energy homeostasis. Its influence on gene expression related to adipogenesis, mitochondrial activity, and glucose transport underscores its long-term potential in metabolic regulation research.
Research Data
| Study / Model | Reported effect |
| Human clinical trials (SURPASS program, T2DM) |
↓ HbA1c (up to -2.4%) and body weight (-12 kg over 40 weeks) vs insulin and Sеmаglutіdе.
|
| Human clinical trials (obesity without diabetes) |
15-22% mean weight loss at 72 weeks; improved lipids and blood pressure.
|
| Comparative RCTs (Tіrzеpаtіdе vs Sеmаglutіdе) |
Higher efficacy in weight and glycemic outcomes; similar tolerability.
|
| NAFLD / NASH models |
↓ hepatic steatosis and transaminases (ALT/AST); improved insulin response and mitochondrial function.
|
| GLP-1/GIP receptor studies (preclinical) |
Dual receptor activation → ↑ insulin secretion, ↓ appetite, ↑ energy efficiency.
|
| Cardiometabolic outcomes (post-hoc human) |
↓ total cholesterol, LDL, triglycerides, CRP; improved insulin sensitivity.
|
| Animal obesity / high-fat diet models |
↓ fat mass, ↑ thermogenesis and glucose tolerance.
|
| Human tolerability studies (Phases 1-3) |
Common mild effects: nausea, diarrhea; no serious hepatic or hematologic effects.
|
| Long-term weight maintenance (extension trials) |
>80% of weight reduction maintained after 88 weeks of continued use.
|
Stack Suggestions
Tіrzеpаtіdе is often stacked with:
- Cagrilintide → synergistic appetite suppression and fat loss via complementary amylin pathway
- 5-Amino-1MQ → enhanced mitochondrial thermogenesis and energy expenditure.
GHK-Cu + BPC-157 stack → supports tissue recovery and insulin sensitivity improvement during weight loss - NAD+ or MOTS-c → improves mitochondrial efficiency and energy balance.
Stacks discussed are for experimental design; not safety/efficacy guidance.
Pen Dosage Chart
| Tіrzеpаtіdе Pen 5 mg | |
| Volume | 1.0 mL |
| mg/mL | 5.0 mg/mL |
| Click-to-Dose | 1 click = 0.05 mg |
| Example(s) | 10 clicks = 0.5 mg; 50 clicks = 2.5 mg |
| Tіrzеpаtіdе Pen 10 mg | |
| Volume | 1.0 mL |
| mg/mL | 10.0 mg/mL |
| Click-to-Dose | 1 click = 0.10 mg |
| Example(s) | 10 clicks = 1 mg; 25 clicks = 2.5 mg |
| Tіrzеpаtіdе Pen 15 mg | |
| Volume | 1.5 mL |
| mg/mL | 10.0 mg/mL |
| Click-to-Dose | 1 click = 0.10 mg |
| Example(s) | 10 clicks = 1 mg; 25 clicks = 2.5 mg |
Dosage & Protocols Variations
Standard Research Protocol
- Dose: 2.5 – 5 mg
- Duration: 8 – 12 weeks
- Frequency: 1× weekly
- Cycle Interval: Optional 2-week pause
- Goal / Description: For basic metabolic modulation studies
Therapeutic Research Protocol
- Dose: 10 – 15 mg
- Duration: 24 – 48 weeks
- Frequency: 1× weekly
- Cycle Interval: Repeat after 1 month
- Goal / Description: Modeled on SURPASS/SURMOUNT designs; for long-term metabolic outcomes
Biohacker Microdosing
- Dose: 0.5 – 1 mg every 3 days
- Duration: 4 – 8 weeks
- Frequency: Every 3 days
- Cycle Interval: Repeat after 1 month
- Goal / Description: Used experimentally to assess mild appetite control and metabolic priming
Maintenance Phase
- Dose: 2.5 – 5
- Duration: Variable
- Frequency: 1× weekly
- Cycle Interval: Continuous
- Goal / Description: Sustain achieved body composition and metabolic balance.
Possible Side Effects
Tіrzеpаtіdе may cause mild and transient gastrointestinal symptoms in some research subjects.
The most common reactions include nausea, diarrhea, and reduced appetite, particularly during initial exposure. Occasional reports include constipation, vomiting, or abdominal discomfort, typically resolving as tolerance develops. Other possible effects include fatigue or transient increases in heart rate during activity.
All effects are reversible and dose-dependent. Proper titration minimizes occurrence.
Product Attributes
- CAS #: 2023788-19-2
- Molecular Formula: C225H348N48O68
- Sequence (AA): Modified 39-amino-acid linear peptide (GLP-1/GIP analog)
- Molecular Weight: ~4813.6 g/mol
- PubChem CID: 155090095
- Half-Life: ~120 hours (≈5 days)
- Synonyms: LY3298176, GLP-1/GIP Dual Agonist, GLP-1T, GIP/GLP-1 RA, Twincretin
- Type: Synthetic peptide analog
- Research Focus: Weight Loss, Metabolic Health, Cardiometabolic Optimization
Scientific References
- Tіrzеpаtіdе once weekly for the treatment of obesity Human RCT
- Tіrzеpаtіdе : a systematic update Review
- Tіrzеpаtіdе versus sеmаglutіdе once weekly in patients with type 2 diabetes Human RCT
- Efficacy and safety of once-weekly tіrzеpаtіdе versus once-daily insulin degludec as add-on to metformin in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial Human RCT
- Dual GIP and GLP-1 receptor agonist Tіrzеpаtіdе improves beta-cell function and insulin sensitivity in type 2 diabetes Human observational
- Effects of novel dual GIP and GLP-1 receptor agonist tіrzеpаtіdе on biomarkers of inflammation and cardiovascular risk in patients with type 2 diabetes Human RCT
- LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept Animal
- A long-term safety study of tіrzеpаtіdе (LY3298176) in participants with type 2 diabetes Human RCT
- Effect of tіrzеpаtіdе versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial Human RCT
- Once-weekly sеmаglutіdе in adolescents with obesity (comparative to tіrzеpаtіdе context) Human RCT
Included In The Box
Every product arrives in a premium, custom-designed PEPTIDE.Power box, engineered for convenience, hygiene, and safe storage in your refrigerator. Inside, you will find everything needed for your full research protocol:
- 1× Disposable Pre-Mixed Injection Pen
- Powered by our proprietary PSM Technology™ – precision stabilization & mixing system for consistent potency
- 10× Ultra-thin Needles (33G, 4 mm)
- 10× Alcohol Pads for sterile preparation
- Internal Stabilizing Foam Insert to prevent shaking during transport
- Instruction Panel printed on the inside of the box for quick reference
- Security Seal Sticker ensuring the package has not been opened or tampered with
Storage
Store the product in a refrigerator at 1 – 7°C immediately upon delivery. To maintain optimal stability, keep the pen away from light, and do not expose it to repeated temperature changes.
Once reconstituted (all our pens come pre-mixed), research compounds remain stable for 6 – 8 weeks under proper refrigeration.
Do not freeze after reconstitution. Always keep the box closed so the pen, needles, and alcohol pads stay clean and protected.
For best results, use the product consistently within the recommended time window and always follow your research protocol.
Delivery
We ship with Next-Day EU Delivery via DHL Express or UPS Express.
All orders are prepared fresh on the day of dispatch, placed in EPS cold-chain transport boxes, and shipped with cooling elements to maintain a stable temperature throughout the journey.
Our logistics process is designed so the package arrives overnight, avoiding customs delays inside the European Union.
Products are shipped from our EU facility, ensuring no import duties, no customs clearance, and always fast and secure delivery.
Payment
Due to the nature of research peptides and the high-risk category assigned by payment processors, credit card companies do not support merchants in this field.
For this reason, we accept bank transfers only.
Within the European Union, SEPA transfers are fast, low-cost, and usually arrive within minutes to a few hours, making the payment process smooth and simple.
Once the transfer is received, your order is prepared immediately and dispatched the same day (cut-off dependent).
This method ensures compliance, security, and continuity of service for all customers across the EU.