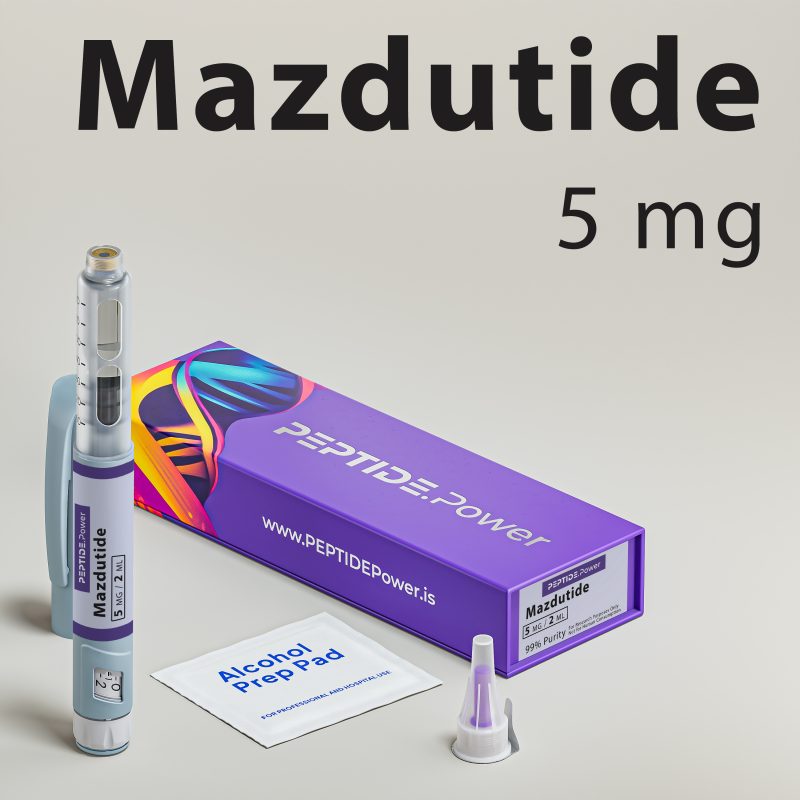
GLP-3R
$153.60 – $403.20Price range: $153.60 through $403.20
GLP-3R - Triple GLP Agonist for Advanced Weight Loss
Description
Rеtаtrutіdе is a synthetic multi-receptor agonist engineered to coordinate satiety signaling, glucose handling, and energy expenditure through concurrent activation of GLP-1R, GIPR, and GCGR. In research models, this triad produces robust weight loss, improved glycemic parameters, and enhanced lipid oxidation, offering a comprehensive platform to study whole-body metabolic remodeling. Research use only – intended for controlled protocols, not for human consumption.
Our formulation is delivered in a stabilized pre-mixed subQ injection pen to support dosing precision and high bioavailability while avoiding reconstitution steps. This format simplifies multi-arm designs where timing and consistency matter, and it aligns with weekly paradigms often used in incretin research.
Clinical status – investigational:
Phase 2 trials have reported large, sustained weight loss and favorable cardiometabolic signals; formal regulatory approvals for rеtаtrutіdе are pending.
Evidence type:
Human RCT ✔ | Observational ✔ | Animal ✔ | In vitro ✔ | Regulatory □
Mechanism of Action
As a unimolecular agonist, Rеtаtrutіdе binds to GLP-1R, GIPR, and GCGR with high affinity, leading to cAMP-mediated signaling cascades that enhance pancreatic beta-cell insulin release, suppress glucagon in hyperglycemia, and increase hepatic fatty acid oxidation. In animal models, this results in upregulated thermogenesis via UCP1 in brown adipose tissue and reduced lipogenesis. In vitro studies confirm synergistic receptor crosstalk, amplifying AMPK activation for improved mitochondrial function and reduced inflammation markers like CRP.
Benefits
- Triple Agonist Mechanism for Comprehensive Metabolic Regulation:
Rеtаtrutіdе is a potent GLP-1, GIP, and glucagon receptor agonist, studied for its multi-pathway activation of energy metabolism. By engaging all three receptors, it enhances insulin secretion, suppresses appetite, and promotes lipid oxidation simultaneously. This triple agonist design provides broader metabolic modulation than single or dual incretin analogs, making it a leading subject in next-generation obesity and diabetes research. - Exceptional Weight Reduction Outcomes:
Clinical and preclinical data show that Rеtаtrutіdе can produce up to 24% body weight reduction over extended treatment periods. This outcome surpasses previous GLP-1 or GLP-1/GIP agonists due to enhanced thermogenesis and fat oxidation mechanisms. Research indicates a sustained and progressive decline in both subcutaneous and visceral fat mass, positioning Rеtаtrutіdе as a new benchmark in metabolic optimization studies. - Marked Reduction in Visceral and Hepatic Fat:
Rеtаtrutіdе has been observed to dramatically reduce liver fat content (over 80% in clinical data) and visceral adiposity in both obese and type 2 diabetic subjects. This effect stems from its ability to activate glucagon receptor-mediated lipid metabolism and enhance mitochondrial β-oxidation, promoting improved liver function and systemic metabolic balance. - Improvement in Glucose Control and Insulin Sensitivity:
Through simultaneous activation of incretin pathways, Rеtаtrutіdе enhances insulin secretion, suppresses glucagon, and improves peripheral glucose uptake. This coordinated metabolic effect results in significant HbA1c reduction and stabilization of fasting glucose levels in research subjects. These findings highlight its potential as one of the most effective glucose-lowering peptides under investigation. - Enhanced Lipid Profile and Cardiovascular Markers:
Rеtаtrutіdе administration has been associated with improved lipid metabolism, including reductions in total cholesterol, LDL, and triglycerides. Studies also report lower systolic blood pressure and inflammatory markers such as CRP, suggesting favorable cardiometabolic outcomes in long-term metabolic studies. - Stimulation of Energy Expenditure and Thermogenesis:
In animal models, Rеtаtrutіdе increases thermogenic activity in brown adipose tissue via glucagon receptor signaling, leading to higher resting energy expenditure. Enhanced mitochondrial respiration and fatty acid oxidation contribute to continuous fat loss even under moderate caloric intake, establishing its potential as a metabolic rate enhancer in research applications. - Reduction of Systemic Inflammation:
Rеtаtrutіdе has been observed to reduce inflammatory cytokines such as TNF-α and IL-6, while improving endothelial function and vascular elasticity. These anti-inflammatory and vasoprotective effects complement its metabolic actions, promoting improved cardiovascular resilience in experimental models of metabolic disease. - Preservation of Lean Body Mass:
Research findings suggest that despite substantial fat loss, Rеtаtrutіdе supports the maintenance of lean muscle tissue. This balance between adipose reduction and muscle preservation reflects its unique energy-partitioning effect, making it valuable for studies exploring body recomposition and athletic performance enhancement. - Liver Function and NAFLD Improvement:
Preclinical and early clinical studies show significant improvements in non-alcoholic fatty liver disease (NAFLD) parameters, including reduced hepatic inflammation and fibrosis. Rеtаtrutіdе’s triple-agonist activation enhances AMPK signaling, reducing lipid accumulation and promoting hepatic regeneration in metabolic liver research. - Synergistic Effects with GLP-1 and Mitochondrial Peptides:
Experimental protocols combining Rеtаtrutіdе with Sеmаglutіdе, MOTS-c, or SS-31 are being explored to enhance mitochondrial bioenergetics, lipid metabolism, and overall metabolic performance. These stacked combinations aim to maximize fat oxidation, reduce inflammation, and sustain high energy efficiency in long-term research models. - Long-Term Metabolic Adaptation and Weight Maintenance:
Follow-up studies indicate that subjects maintained over 80% of their weight loss after extended observation periods, even after discontinuation. This sustained adaptation reflects durable improvements in metabolic set-point, appetite control, and mitochondrial efficiency, marking Rеtаtrutіdе as a highly promising agent for advanced metabolic research.
Research Data
| Study/model | Reported effect |
| Human clinical trials – Phase 2, obesity (no diabetes) |
~24% mean weight loss at 48 weeks with weekly dosing – highest reported among incretin agonists to date.
|
| Human clinical trials – Phase 2, T2DM |
HbA1c ↓ up to ~2.2% with significant weight reduction (~17%) vs placebo, plus lipid and insulin-sensitivity improvements.
|
| Triple agonist mechanism models |
GLP-1/GIP/GCGR activation → ↑ energy expenditure, ↑ fat oxidation, ↓ appetite and lipogenesis.
|
| Rodent diet-induced obesity |
Sharp declines in fat mass and visceral adiposity; ↑ thermogenesis and UCP1 expression in brown adipose tissue.
|
| NAFLD models |
↓ steatosis, inflammation, and fibrosis via lipid-metabolism modulation and AMPK signaling.
|
| Cardiometabolic endpoints (Phase 2 extension) |
↓ systolic BP (~9 mmHg), ↓ CRP and triglycerides; improved arterial elasticity.
|
| Tolerability across trials |
Most common: nausea and diarrhea (generally transient); no major cardiac or hepatic safety signals observed to date.
|
Stack Suggestions
- Rеtаtrutіdе + Cagrilintide – appetite control plus energy-expenditure focus for body-weight endpoints.
Rationale: Amylin-analog synergy on satiety with triple-agonist metabolic activation. - Rеtаtrutіdе + 5-Amino-1MQ – lipid-flux modulation with NNMT inhibition alongside incretin-glucagon signaling.
Rationale: Encourages fat-use preference while reducing lipogenesis signals. - Rеtаtrutіdе + NAD+ – cellular-energy and mitochondrial-support cofactor during rapid metabolic shifts.
Rationale: Supports redox balance in intensive weight-loss paradigms. - Rеtаtrutіdе + BPC-157/TB-500 (recovery contexts) – tissue comfort and adherence during lifestyle interventions.
Rationale: Soft-tissue support may reduce musculoskeletal barriers to activity.
Note: Stacks are for experimental design only – not safety or efficacy guidance.
Pen Dosage Chart
| Rеtаtrutіdе Pen 5 mg | |
| Volume | 1.0 mL |
| mg/mL | 5.0 mg/mL |
| Click-to-Dose | 1 click = 0.05 mg |
| Example(s) | 10 clicks = 0.5 mg; 50 clicks = 2.5 mg |
| Rеtаtrutіdе Pen 10 mg | |
| Volume | 1.0 mL |
| mg/mL | 10.0 mg/mL |
| Click-to-Dose | 1 click = 0.10 mg |
| Example(s) | 10 clicks = 1 mg; 25 clicks = 2.5 mg |
| Rеtаtrutіdе Pen 15 mg | |
| Volume | 1.5 mL |
| mg/mL | 10.0 mg/mL |
| Click-to-Dose | 1 click = 0.10 mg |
| Example(s) | 10 clicks = 1 mg; 25 clicks = 2.5 mg |
Dosage & Protocols Variations
Standard Research Protocol
- Dose: 2 – 4 mg weekly, titrate to 12 mg
- Duration: 20 – 48 weeks
- Frequency: 1× weekly
- Cycle Interval: 20 – 48 weeks on, 4 – 8 weeks off, then reassess
- Goal / Description: Gradual weight reduction, improved glycemic markers
Therapeutic Research Protocol
- Dose: 4 mg weekly, up to 12 mg
- Duration: 16 – 52 weeks
- Frequency: 1× weekly
- Cycle Interval: 12 – 16 weeks on, 6 weeks off
- Goal / Description: Larger weight-loss magnitude, liver-fat decline
Biohacker
- Dose: 1 mg, escalate by 1 – 2 mg/week
- Duration: 10 – 14 weeks
- Frequency: 1× weekly
- Cycle Interval: 10 – 14 weeks on, 4 – 6 weeks off
- Goal / Description: Personalized balance of effect vs GI side effects
Glucose-Focus Protocol
- Dose: 0.25 – 1.0 mg weekly
- Duration: 8 – 10 weeks
- Frequency: 1× weekly
- Cycle Interval: 8 – 10 weeks on, 4 weeks off
- Goal / Description: Improved fasting/postprandial control
Possible Side Effects
Rеtаtrutіdе, as a research peptide mimicking incretin hormones, may induce various side effects in experimental models, primarily related to its influence on gastrointestinal and metabolic systems. These effects are often dose-dependent and more prominent during initial administration or rapid escalation. It’s crucial to monitor subjects closely, as subcutaneous delivery can sometimes cause localized reactions.
Nausea: Commonly observed, especially at higher doses like 8 – 12 mg, manifesting as mild to moderate queasiness that may disrupt feeding behaviors in models. This arises from delayed gastric emptying via GLP-1 receptor activation. It typically subsides after 2 – 4 weeks as adaptation occurs.
Vomiting: In some cases, particularly with abrupt dose increases, vomiting may occur shortly after injection. This is linked to central nervous system signaling from the area postrema. Frequency decreases with slower titration protocols.
Diarrhea or Constipation: Alterations in bowel habits are frequent, with diarrhea stemming from increased intestinal motility and constipation from reduced food intake. These can alternate and may require hydration adjustments in long-term studies.
Fatigue: A sense of lethargy or reduced activity levels is reported early on, possibly due to caloric restriction effects or transient metabolic shifts. It often resolves as energy homeostasis stabilizes.
Increased Heart Rate: Mild tachycardia, especially during exertion, has been noted in cardiovascular monitoring, attributed to glucagon’s stimulatory effects. No severe arrhythmias in trials, but pulse tracking is advised.
Injection Site Reactions: Subcutaneous administration may lead to redness, swelling, or itching at the site, typically resolving within hours. Rotating injection areas minimizes this.
Most side effects are transient and manageable through dose adjustments or supportive measures in research settings. However, prolonged exposure warrants vigilance for potential gallbladder-related issues or muscle loss, though rare in controlled protocols.
Product Attributes
- CAS #: 2381089-83-2
- Molecular Formula: C221H342N46O68
- Sequence (AA): YXQGTFTSDYSILLDKKAQXAFIEYLLEGGPSSGAPPPS (X = Aib)
- Molecular Weight: 4731.33 g/mol
- PubChem CID: 171390338
- Half-Life: ~6 days
- Synonyms: LY3437943, Triple G incretin-glucagon agonist – GLP-1/GIP/GCGR agonist – TRI-agonist, RETA, Retaglutide, Retatratide, Retalutide
- Type: Synthetic research peptide – multi-receptor agonist
- Research Focus: Weight Loss, Metabolic Health, Liver Fat
Scientific References
- Triple-hormone-receptor agonist rеtаtrutіdе for obesity Human RCT
- Efficacy and safety of rеtаtrutіdе, a novel GLP-1, GIP, and glucagon receptor agonist, in adults with obesity: a randomised, placebo-controlled, phase 2 trial Human RCT
- Rеtаtrutіdе – a game changer in obesity pharmacotherapy Observational
- Unleashing the power of rеtаtrutіdе: a possible triumph over obesity and its comorbidities Animal
- Effects of once-weekly subcutaneous Rrеtаtrutіdе on weight and metabolic markers in adults with obesity: a randomized phase 2 trial Human RCT
- Effects of rеtаtrutіdе on body composition in people with type 2 diabetes: a post hoc analysis of a randomised, double-blind, placebo-controlled phase 2 trial Human RCT
- A review of an investigational drug Rеtаtrutіdе, a novel triple agonist agent for the treatment of obesity Animal
- Rеtаtrutіdе, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA Human RCT
- Unleashing the power of rеtаtrutіdе: a possible triumph over obesity and its comorbidities Animal
- Triple-hormone-receptor agonist rеtаtrutіdе for obesity Human RCT
Included In The Box
Every product arrives in a premium, custom-designed PEPTIDE.Power box, engineered for convenience, hygiene, and safe storage in your refrigerator. Inside, you will find everything needed for your full research protocol:
- 1× Disposable Pre-Mixed Injection Pen
- Powered by our proprietary PSM Technology™ – precision stabilization & mixing system for consistent potency
- 10× Ultra-thin Needles (33G, 4 mm)
- 10× Alcohol Pads for sterile preparation
- Internal Stabilizing Foam Insert to prevent shaking during transport
- Instruction Panel printed on the inside of the box for quick reference
- Security Seal Sticker ensuring the package has not been opened or tampered with
Storage
Store the product in a refrigerator at 1 – 7°C immediately upon delivery. To maintain optimal stability, keep the pen away from light, and do not expose it to repeated temperature changes.
Once reconstituted (all our pens come pre-mixed), research compounds remain stable for 6 – 8 weeks under proper refrigeration.
Do not freeze after reconstitution. Always keep the box closed so the pen, needles, and alcohol pads stay clean and protected.
For best results, use the product consistently within the recommended time window and always follow your research protocol.
Delivery
We ship with Next-Day EU Delivery via DHL Express or UPS Express.
All orders are prepared fresh on the day of dispatch, placed in EPS cold-chain transport boxes, and shipped with cooling elements to maintain a stable temperature throughout the journey.
Our logistics process is designed so the package arrives overnight, avoiding customs delays inside the European Union.
Products are shipped from our EU facility, ensuring no import duties, no customs clearance, and always fast and secure delivery.
Payment
Due to the nature of research peptides and the high-risk category assigned by payment processors, credit card companies do not support merchants in this field.
For this reason, we accept bank transfers only.
Within the European Union, SEPA transfers are fast, low-cost, and usually arrive within minutes to a few hours, making the payment process smooth and simple.
Once the transfer is received, your order is prepared immediately and dispatched the same day (cut-off dependent).
This method ensures compliance, security, and continuity of service for all customers across the EU.